Total synthesis of (−)-dysiherbaine†
Abstract
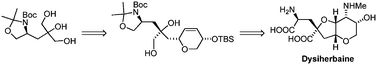
The enantioselective synthesis of (−)-dysiherbaine (1) has been established with efficiency via a unique synthetic strategy involving the desymmetrization of 2-substituted glycerol to install a quaternary chiral carbon, which induces further stereochemistry in the bicyclic perhydrofuropyran through mercuriocyclization and

 Please wait while we load your content...
Please wait while we load your content...