Pd-catalyzed domino carbonylative–decarboxylative allylation: an easy and selective monoallylation of ketones†
Abstract
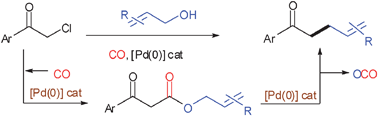
In the presence of an allyl alcohol, α-chloroacetophenones undergo an allyloxycarbonylation reaction followed by in situ decarboxylative

 Please wait while we load your content...
Please wait while we load your content...