Organocatalytic asymmetric domino sulfa-Michael–aldol reactions of 2-mercaptobenzaldehyde with α,β-unsaturated N-acylpyrazoles for the construction of thiochromane†
Abstract
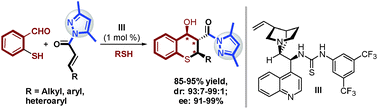
An efficient protocol for the direct construction of bioactive thiochromanes was developed via a catalytic asymmetric cascade sulfa-Michael–

 Please wait while we load your content...
Please wait while we load your content...