Et3N-promoted tandem ring-opening reaction of N-tosylaziridines with terminal alkynoates: a straightforward synthesis of functionalized enamines†
Abstract
A tandem ring-opening reaction of N-tosylaziridines with terminal alkynoates promoted by Et3N has been described. A variety of N-tosylaziridines reacted with terminal alkynoates to give functionalized
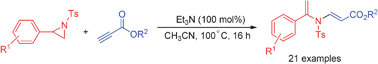

 Please wait while we load your content...
Please wait while we load your content...