PdCl2(dppf)-catalyzed in situ coupling of 2-hydroxypyridines with aryl boronic acids mediated by PyBroP and the one-pot chemo- and regioselective construction of two distinct aryl–aryl bonds†
Abstract
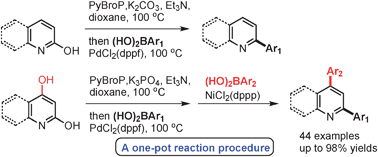
We present a PdCl2(dppf)-catalyzed synthesis of 2-arylated pyridine derivatives via the in situ coupling of 2-OH pyridines and

 Please wait while we load your content...
Please wait while we load your content...