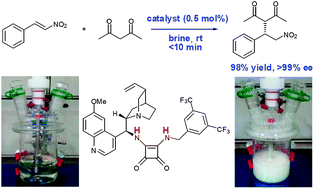
Hydrogen bonding mediated enantioselective organocatalysis in brine: significant rate acceleration and enhanced stereoselectivity in enantioselective Michael addition reactions of 1,3-dicarbonyls to β-nitroolefins†
Abstract
Brine provides remarkable rate acceleration and a higher level of stereoselectivity over organic

 Please wait while we load your content...
Please wait while we load your content...