Stereospecificity in the Au-catalysed cyclisation of monoallylic diols. Synthesis of (+)-isoaltholactone†
Abstract
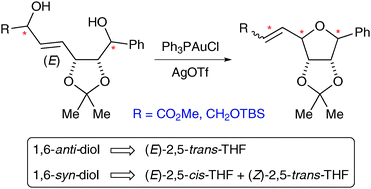
We describe a concise synthesis of (+)-isoaltholactonevia a Au-catalysed cyclisation of a monoallylic diol to form the tetrahydrofuranyl ring. Analogous cyclisations show that the stereochemical outcome is dictated by the stereochemistry of the

 Please wait while we load your content...
Please wait while we load your content...