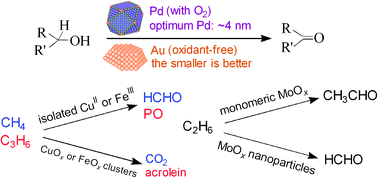
The size of the active phase is one of the most important factors in determining the catalytic behaviour of a heterogeneous catalyst. This Feature Article focuses on the size effects in two types of reactions, i.e., the metal nanoparticle-catalysed dehydrogenation of alcohols and the metal oxide nanocluster-catalysed selective oxidation of hydrocarbons (including the selective oxidation of methane and ethane and the epoxidation of propylene). For Pd or Au nanoparticle-catalysed oxidative or non-oxidative dehydrogenation of alcohols, the size of metal nanoparticles mainly controls the catalytic activity by affecting the activation of reactants (either alcohol or O2). The size of oxidic molybdenum species loaded on SBA-15 determines not only the activity but also the selectivity of oxygenates in the selective oxidation of ethane; highly dispersed molybdenum species are suitable for acetaldehyde formation, while molybdenum oxide nanoparticles exhibit higher formaldehyde selectivity. CuII and FeIII isolated on mesoporous silica are highly efficient for the selective oxidation of methane to formaldehyde, while the corresponding oxide clusters mainly catalyse the complete oxidation of methane. The lattice oxygen in iron or copper oxide clusters is responsible for the complete oxidation, while the isolated CuI or FeII generated during the reaction can activate molecular oxygen forming active oxygen species for the selective oxidation of methane. Highly dispersed CuI and FeII species also function for the epoxidation of propylene by O2 and N2O, respectively. Alkali metal ions work as promoters for the epoxidation of propylene by enhancing the dispersion of copper or iron species and weakening the acidity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...