Expedient entry to the piperazinohydroisoquinoline ring system using a sequential Ugi/Pictet–Spengler/reductive methylation reaction protocol†
Abstract
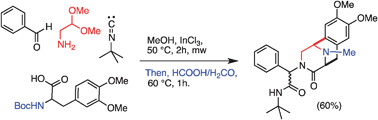
An expedient entry to the piperazinohydroisoquinoline ring system present in the tetrahydroisoquinoline antitumor

 Please wait while we load your content...
Please wait while we load your content...