The (4+3)-cycloaddition reaction: heteroatom-substituted allylic cations as dienophiles
Abstract
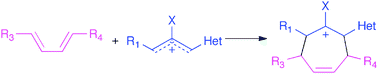
The (4+3)-cycloaddition of allylic cations to dienes is a powerful method for the direct synthesis of seven-membered rings. Recent developments in this area have included new methods for the generation of allylic cations, diastereoselective and

 Please wait while we load your content...
Please wait while we load your content...