Product-selectivity control by the nature of the catalyst: Lewis acid-catalyzed selective formation of ring-fused tetrahydroquinolines and tetrahydroazepines via intramolecular redox reaction†
Abstract
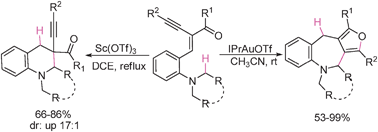
Selective synthesis of ring-fused tetrahydroquinolines and tetrahydroazepines from the same starting materials was achieved by subtle use of the oxophilic

 Please wait while we load your content...
Please wait while we load your content...