One-pot asymmetric cyclocarbohydroxylation sequence for the enantioselective synthesis of functionalised cyclopentanes†
Abstract
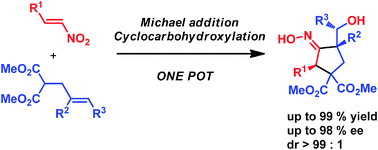
A new method has been developed for the enantioselective synthesis of highly functionalised cyclopentanes bearing up to three stereogenic centres with very high stereoselectivity. This one-pot process combines an enantioselective organocatalytic

 Please wait while we load your content...
Please wait while we load your content...