Enantioselective organocatalytic phospha-Michael reaction of α,β-unsaturated ketones†
Abstract
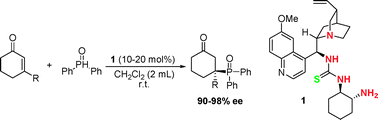
Enantioselective organocatalytic phospha-Michael reaction of α,β-unsaturated ketones and diaryl phosphine oxides has been developed for the first time employing multifunctional organocatalysts. Optically active products bearing quaternary chiral

 Please wait while we load your content...
Please wait while we load your content...