Cinchona-based phase-transfer catalysts for asymmetric synthesis
Abstract
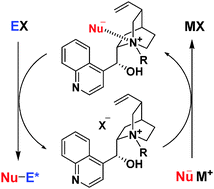
Phase-transfer catalysis is one of the most useful methodologies for practical syntheses given its operational simplicity and mild reaction conditions that enable its application in industrial processes.

 Please wait while we load your content...
Please wait while we load your content...