Formation of pentacyclic structures by a domino sequence on cyclic enamides†
Abstract
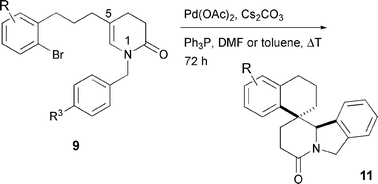
Novel spiropentacyclic compounds 11 were obtained from cyclic enamides of type 9, featuring a (2-bromophenyl)propyl substituent in the 5-position, through a Pd-mediated domino sequence taking place via

 Please wait while we load your content...
Please wait while we load your content...