Reaction of 3,5-Pri2Ar*Fe(η6-C6H6)(3,5-Pri2Ar* = C6H1-2,6-(C6H2-2,4,6-Pri3)2-3,5-Pri2) with N3C6H3-2,6-Mes2 (Mes = C6H2-2,4,6-Me3) afforded the dimeric iron(II) amido/aryl complex {CH2C6H2-2(C6H3-2-N(H)FeAr*-3,5-Pri2)-3,5-Me2}2 (1) which arises viamethyl hydrogen abstraction by nitrogen and dimerization of the radical via C–C bond formation; in contrast, reaction of 3,5-Pri2Ar*Fe(η6-C6H6) with N3(1-Ad) (1-Ad = 1-adamantanyl) gave the iron(V) bis(imido) complex 3,5-Pri2Ar*Fe{N(1-Ad)}2 (2).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
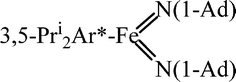

 Please wait while we load your content...
Please wait while we load your content...