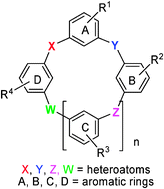
Heterocalixaromatics, the heteroatom bridged calix(hetero)arenes, have been emerging as new generation macrocyclic host molecules in supramolecular chemistry recently. Being different from the conventional calixarenes in which the aromatic rings are linked by methylene units, heterocalixaromatics assemble various aromatic rings by different heteroatoms. Owning to the intrinsic nature of heteroatoms that can adopt different electronic configurations to form various degrees of conjugation with their neighboring aromatic rings, heterocalixaromatics exhibit unique structural features and versatile recognition properties in comparison to conventional calixarenes. This feature article highlights recent advances in the synthesis, functionalization, structure and molecular recognition of nitrogen- and/or oxygen-bridged calixaromatics, with a primary focus on our own work.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...