One-pot synthesis of a pentasaccharide with antibiotic activity against Helicobacter pylori†
Abstract
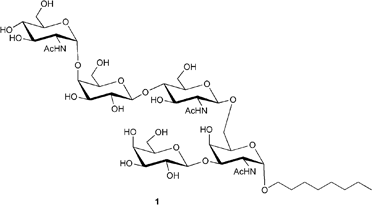
A pentasaccharide that contains the α-1,4-GlcNAc mucin core two-branched O-glycan has been synthesized by a one-pot, two-step glycosylation strategy; this particular

 Please wait while we load your content...
Please wait while we load your content...