Proline organocatalysis as a new tool for the asymmetric synthesis of ulosonic acid precursors†
Abstract
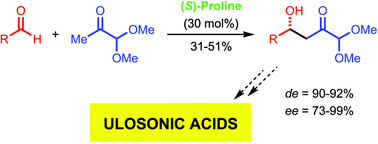
PEP and aldolase mimicry is the key for a direct organocatalytic entry to precursors of ulosonic acids, biomolecules of enormous importance in biology, chemistry and medicine; in the key aldol reaction the dimethylacetal of

 Please wait while we load your content...
Please wait while we load your content...