Tandem Staudinger–azaWittig mediated ring expansion: rapid access to new isofagomine-tetrahydroxyazepane hybrids†
Abstract
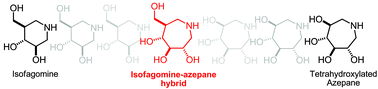
New seven-membered iminosugars with potent and selective inhibition towards glycosidases have been prepared as 1-N-iminosugar homologues via a tandem Staudinger–azaWittig mediated ring expansion.

 Please wait while we load your content...
Please wait while we load your content...