A normal equilibrium isotope effect for oxidative addition of H2 to (η6-anthracene)Mo(PMe3)3†
Abstract
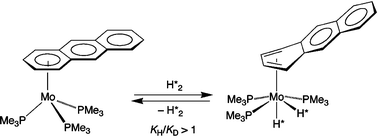
Oxidative addition of H2 and D2 to the anthracene complex (η6-AnH)Mo(PMe3)3 giving (η4-AnH)Mo(PMe3)3X2 (X = H, D) is characterized by a normal equilibrium isotope effect (KH/KD > 1) at temperatures close to ambient; calculations on (η4-AnH)Mo(PH3)3H2 indicate that this is a consequence of relatively low energy Mo–H vibrational modes.

 Please wait while we load your content...
Please wait while we load your content...