68Zn isotope exchange experiments reveal an unusual kinetic lability of the metal ions in the di-zinc form of IMP-1 metallo-β-lactamase†
Abstract
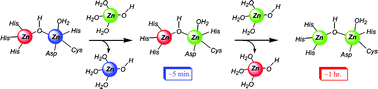
The apparently paradoxical behaviour of facile exchange (kinetic lability) of tightly bound (thermodynamic stability)

 Please wait while we load your content...
Please wait while we load your content...