Lipase-catalyzed domino kinetic resolution of α-hydroxynitrones/intramolecular 1,3-dipolar cycloaddition: a concise asymmetric total synthesis of (−)-rosmarinecine†
Abstract
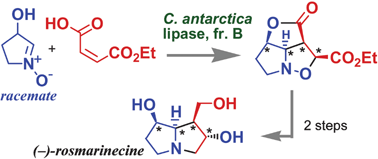
The title domino reactions were developed to directly provide tetrahydrofuro[3,4-c]isoxazole derivatives (5 and 9) in ≥90% ee from racemic α-hydroxynitrones (2 and 6), which were used in the concise asymmetric total synthesis of (−)-rosmarinecine 10.

 Please wait while we load your content...
Please wait while we load your content...