Preparation of cyclic alkenylmagnesium reagents via an iodine/magnesium exchange†
Abstract
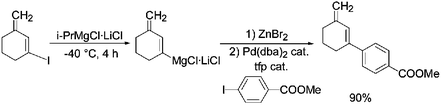
The reaction of various cyclic alkenyl iodides with i-PrMgCl·LiCl produces the corresponding alkenylmagnesium reagents under mild conditions. After reaction with various electrophiles, like allylic

 Please wait while we load your content...
Please wait while we load your content...