Novel formation and use of a Nicholas carbocation in the synthesis of highly substituted tetrahydrofurans†
Abstract
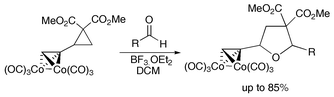
The first formation of a Nicholas carbocation through cleavage of a carbon–carbon σ bond has allowed the preparation of highly substituted tetrahydrofurans in a formal dipolar cycloaddition reaction.

 Please wait while we load your content...
Please wait while we load your content...