The electronic structure of nitrilimines revisited†
Abstract
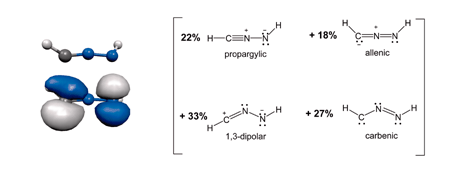
A combination of density-functional theory and natural resonance theory has been used to show that a complete description of the electronic structure of nitrilimines, R1CNNR2, requires four resonance structures (propargylic, allenic, 1,3-dipolar and carbenic); appropriate substituents were shown to enhance the

 Please wait while we load your content...
Please wait while we load your content...