Reactivity of an anionic Pd(ii) metallacycle with CH2X2 (X = Cl, Br, I): formal insertion of methylene into a Pd–Caryl bond†
Abstract
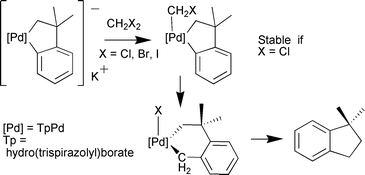
Whereas the reaction of the anionic ![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) d(CH2CMe2-o-C
d(CH2CMe2-o-C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) 6H4)(κ2-Tp)}] with CH2Cl2 leads to the isolation of the stable Pd(IV) chloromethyl complex [P
6H4)(κ2-Tp)}] with CH2Cl2 leads to the isolation of the stable Pd(IV) chloromethyl complex [P![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) d(CH2CMe2-o-C
d(CH2CMe2-o-C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) 6H4)(κ3-Tp)(CH2Cl)], the analogous reactions with CH2Br2 and CH2I2 give rise to the six membered metallacycles [P
6H4)(κ3-Tp)(CH2Cl)], the analogous reactions with CH2Br2 and CH2I2 give rise to the six membered metallacycles [P![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) d(CH2CMe2-o-C6H4(C
d(CH2CMe2-o-C6H4(C![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) H2))(κ3-Tp)X]
(X = Br or I), as a result of the formal insertion of CH2 into the Pd–Caryl bond.
H2))(κ3-Tp)X]
(X = Br or I), as a result of the formal insertion of CH2 into the Pd–Caryl bond.

 Please wait while we load your content...
Please wait while we load your content...