Total synthesis of (−)-dysibetaine via a nitrenium ion cyclization–dienone cleavage strategy†
Abstract
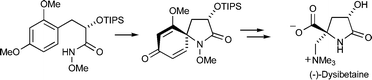
The diastereoselective total synthesis of the marine natural product (−)-dysibetaine is reported. The key steps in this venture are i) a diastereoselective nitrenium ion spirocyclization, which serves to generate the pyrrolidinone ring and quaternary stereocenter of the target, and ii) use of the 2-methoxycyclohexa-2,5-dienone ring formed during

 Please wait while we load your content...
Please wait while we load your content...