Fast racemisation and slow epimerisation of laterally lithiated amides: stereochemical evidence for the mechanism of inversion of amide-substituted benzyllithiums
Abstract
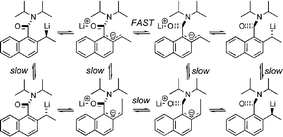
Tertiary 1-naphthamides racemise much more slowly than their laterally lithiated derivatives, and the relative rates of racemisation and epimerisation of these derivatives indicate that the lithium-bearing stereogenic centre inverts via a “conducted tour” mechanism, in which the lithium

 Please wait while we load your content...
Please wait while we load your content...