Composite hopanoid biosynthesis in Zymomonas mobilis: N-acetyl-d-glucosamine as precursor for the cyclopentane ring linked to bacteriohopanetetrol
Abstract
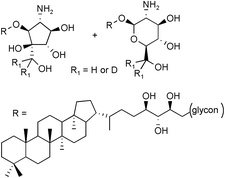
A selective labelling of the two major composite hopanoids of Z. mobilis with deuterated N-acetyl-D-glucosamine showed that this carbohydrate is a common precursor of the glucosamine or the cyclopentitol moieties respectively linked to bacteriohopanetetrol by a glycosidic or an ether bond.

 Please wait while we load your content...
Please wait while we load your content...