Conformational studies on phenyl thioglycosides: a remote effect on disaccharide linkage by phenyl aglycons attenuates recognition of galabiosides by a bacterial adhesin
Abstract
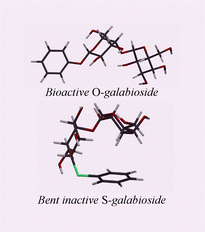
Phenyl S-galabiosides display altered conformational properties, as compared to phenyl O-galabiosides, characterised by a remote effect on the galabiose intersaccharidic

 Please wait while we load your content...
Please wait while we load your content...