Asymmetric Michael addition of formaldehyde N,N-dialkylhydrazones to alkylidene malonates
Abstract
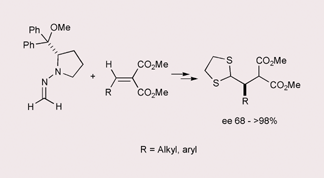
Enantiopure formaldehyde N,N-dialkylhydrazones 1 smoothly react with prochiral alkylidene malonates 2 in the presence of MgI2 to afford the corresponding Michael adducts 3 in excellent yields and good diastereoselectivities; direct racemization-free BF3·OEt2-catalyzed thiolysis of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond affords the corresponding
N bond affords the corresponding

 Please wait while we load your content...
Please wait while we load your content...