Self-assisted tandem Michael-aldol reactions of α,β-unsaturated ketones with aldehydes
Abstract
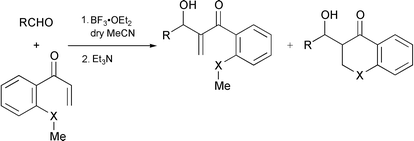
The tandem Michael-aldol reaction of 1-[2-(methylsulfanyl)phenyl]prop-2-en-1-one (1) or the seleno congener 4 with p-nitrobenzaldehyde in the presence of BF3·Et2O gave the Baylis–Hillman adduct 2 or 5 and onium salt 3 or 6, respectively, and selenochromanone 7 from 4.

 Please wait while we load your content...
Please wait while we load your content...