Orthogonal enzymatic reactions for rapid crosslinking and dynamic tuning of PEG–peptide hydrogels
Abstract
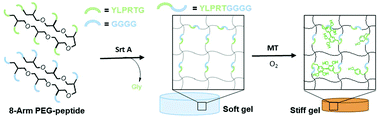
Stiffening of the extracellular matrix is a hallmark in cancer progression, embryonic development, and wound healing. To mimic this dynamic process, our work explored orthogonal enzymatic reactions capable of modulating the properties of poly(ethylene glycol) (PEG)–peptide hydrogels. A hepta-mutant bacterial transpeptidase sortase A (SrtA7M) was used to ligate two PEG–peptide macromers (i.e., PEG-YLPRTG and NH2-GGGG-PEG) into a primary hydrogel network. The hydrogels were dynamically stiffened using mushroom tyrosinase (MT), which oxidized tyrosine residues into di-tyrosine and led to increased matrix stiffness. After confirming the expression and enhanced catalytic activity of SrtA7M, we investigated the cytocompatibility of the enzymatic reaction with a mouse insulinoma cell line, MIN6. In addition, we altered peptide substrate concentrations and evaluated their influence on primary hydrogel network properties and MT-triggered stiffening. Using a pancreatic cancer cell line, COLO-357, the effect of MT-triggered stiffening on spheroid formation was investigated. We found that cell spheroids formed in hydrogels that were exposed to MT were significantly smaller than spheroids formed without MT incubation, suggesting that matrix stiffening played a crucial role in the sizes of cancer cell spheroids. Through utilizing highly specific and orthogonal enzymatic reactions, this hydrogel platform permits rapid and mild in situ cell encapsulation, as well as dynamic control of matrix stiffness for investigating the role of matrix stiffening on cell fate processes.


 Please wait while we load your content...
Please wait while we load your content...