Bioinspired onion epithelium-like structure promotes the maturation of cardiomyocytes derived from human pluripotent stem cells†
Abstract
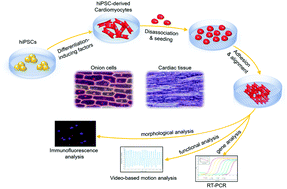
Organized cardiomyocyte alignment is critical to maintain the mechanical properties of the heart. In this study, we present a new and simple strategy to fabricate a biomimetic microchip designed with an onion epithelium-like structure and investigate the guided behavior of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) on the substrate. The hiPSC-CMs were observed to be confined by the three dimensional surficial features morphologically, analogous to the in vivo microenvironment, and exhibited an organized anisotropic alignment on the onion epithelium-like structure with good beating function. The calcium imaging of hiPSC-CMs demonstrated a more mature Ca2+ spark pattern as well. Furthermore, the expression of sarcomere genes (TNNI3, MYH6 and MYH7), potassium channel genes (KCNE1 and KCNH2), and calcium channel genes (RYR2) was significantly up-regulated on the substrate with an onion epithelium-like structure instead of the surface without the structure, indicating a more matured status of cardiomyocytes induced by this structure. It appears that the biomimetic micropatterned structure, analogous to in vivo cellular organization, is an important factor that might promote the maturation of hiPSC-CMs, providing new biological insights to guide hiPSC-CM maturation by biophysical factors. The established approach may offer an effective in vitro model for investigating cardiomyocyte differentiation, maturation and tissue engineering applications.


 Please wait while we load your content...
Please wait while we load your content...