Immobilising proteins on silica with site-specifically attached modified silaffin peptides†
Abstract
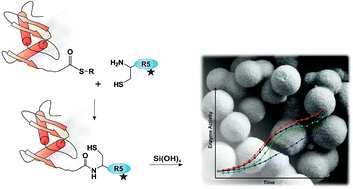
Immobilisation of proteins on solid supports such as silica is commonly applied to improve performance of enzymes under detrimental conditions and to allow enzyme recycling. Silica biomineralisation processes occurring in nature have recently inspired approaches towards mild, biomimetic silica formation. In diatoms, complex posttranslationally modified silaffin peptides are directly involved in formation and patterning of silica cell walls. Here, chemically modified silaffin peptides are used to establish a novel strategy for silica immobilisation of target proteins. Silaffin variants carrying different modifications are covalently linked to eGFP and thioredoxin using expressed protein ligation. Covalent eGFP- and thioredoxin-silaffin conjugates are able to efficiently precipitate silica and control silica properties by choice of different silaffin modifications leading to functional encapsulation of these proteins in silica particles. Covalent protein-silaffin conjugates lead to a distinctly more efficient and homogenous encapsulation of proteins in silica, superior to random protein entrapment resulting from simple co-precipitation. Silica-immobilised proteins are confirmed to be fully active and stabilised against denaturation.

 Please wait while we load your content...
Please wait while we load your content...