Quantitative measurements of thermodynamics and kinetics of polythiophene–DNA complex formation in DNA detection†
Abstract
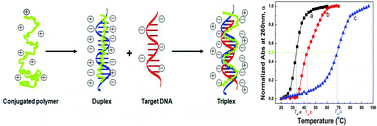
Cationic polythiophene derivatives have been used as unique optical probes for various biosensing applications with great success, because their optical responses are sensitive to the conformational changes from binding to single-stranded DNA (ssDNA) to binding to double-stranded DNA (dsDNA). It has long been suggested that the binding of cationic polymers to DNA has a major impact on the thermodynamics and kinetics of DNA hybridization; yet a quantitative assessment is lacking. We report here a systematic study to quantitatively measure the thermodynamic binding constants and hybridization rates of DNA duplexes in the presence/absence of cationic polythiophene. Our results show that the dissociation constant of dsDNA in the presence of polythiophene is three orders of magnitude smaller than that in the absence of polymers. The formation of the DNA/polymer triplex is also much slower than double helical DNA formation, suggesting a rate-limiting polymer–ssDNA dissociation step prior to DNA hybridization. The hybridization rate constant is further slowed down when polymer/DNA hybridization occurs on a solid surface due to steric hindrance. Having a means to quantitatively assess the DNA hybridization efficiency in the presence of cationic polymers, we were able to improve the DNA sensing performance through a combined tuning of reaction temperature and time.

 Please wait while we load your content...
Please wait while we load your content...