A facile strategy to prepare redox-responsive amphiphilic PEGylated prodrug with high drug loading content and low critical micelle concentration†
Abstract
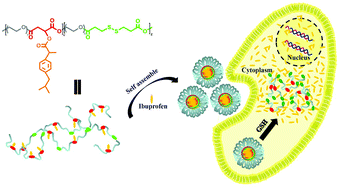
A redox-responsive amphiphilic polymeric prodrug was synthesized in a facile way by polycondensation of oligo(ethylene glycol) with dicarboxylic acids including malic acid and 3,3′-dithiodipropionic acid , followed by esterification with ibuprofen, which was used as a model drug. Because of its amphiphilic nature and relatively high molecular weight, this polymeric prodrug can form stable micelles in aqueous media with a low critical micellar concentration (CMC). Free ibuprofen molecules can be steadily incorporated into the core of these micelles with a surprisingly high loading content (38.9 wt%), owing to hydrophobic interaction and π–π stacking with the ibuprofen moieties in the copolymer. The in vitro release results indicate that there was a relatively slow and sustained release of the conjugated ibuprofen moieties, while encapsulated ibuprofen molecules showed a rapid release. Furthermore, for both the conjugated ibuprofen and the encapsulated ibuprofen there was an accelerated release in the presence of 10 mM DL-dithiothreitol due to cleavage of the disulfide bonds, which lead to disassociation of the micelles. Notably, this prodrug was revealed to have excellent cell compatibilities via a cell counting kit-8 (CCK-8) assay. Confocal laser scanning microscope observations indicated that the micelles based on the polymeric prodrug can be taken up quickly by cells and present a redox-responsive drug release in cytoplasm. This kind of polymeric nanocarrier with a high drug loading content, low CMC, excellent biocompatibility and rapid response to a reductive environment may have tremendous scope in the area of controlled drug delivery.

 Please wait while we load your content...
Please wait while we load your content...