A fluorescent colorimetric azo dye based chemosensor for detection of S2− in perfect aqueous solution and its application in real sample analysis and building a molecular logic gate†
Abstract
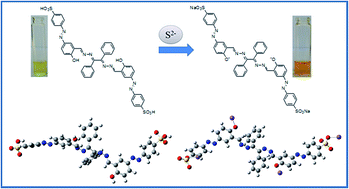
A simple azo-dye based bis-Schiff base chemosensor, L, has been designed, synthesized and characterized by NMR and IR spectroscopy, ESI-MS and elemental analysis. The chemosensor L can successfully detect sulfide ions both visually and spectro-photometrically in perfect aqueous medium as well as in an organic–aqueous mixture through a de-protonation mechanism. The detection limit reaches 16 μM in pure aqueous medium (1.7 μM by UV-vis titration and 3.35 × 10−7 M by fluorescence titration in a 1 : 1 DMSO–H2O mixture), which is the lowest detection limit ever reported in the family of Schiff base chemosensors for sulfide ions. Other interfering anions (F−, Cl−, Br−, HSO3−, N3−, PO43−, NO3−, OAc−, CO32− and CN−) exhibited no interference for the detection of sulfide ions. The mechanism of interaction between L and sulphide ions has further been confirmed by DFT studies. The chemosensor L has also been applied for real sample analysis and in building a molecular logic gate.


 Please wait while we load your content...
Please wait while we load your content...