A new ‘Turn-on’ PET-CHEF based fluorescent sensor for Al3+ and CN− ions: applications in real samples†
Abstract
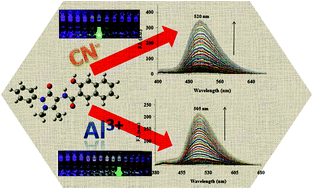
An antipyrine and naphthoic acid-based hybrid molecular architect was synthesized and its photophysical behavior towards various cations and anions was further investigated. Probe 1 was used for the selective estimation of Al3+ ions and signalled the binding event through the formation of a new absorption band at 405 nm and a ‘turn-on’ fluorescence band at 505 nm via the CHEF effect, and restricting the PET phenomenon with the lowest detection limit of 28 nM. Probe 1 demonstrated selective estimation of CN− and OH− ions among all other anions by forming a new absorption band at 400 nm and a fluorescence ‘turn-on’ at 520 nm with the lowest detection limit of 77 nM. Aluminium concentration of up to 7.4–8.6 ppm was also detected by probe 1 in sewage water. All the results were correlated by 1H NMR titrations and theoretical studies.


 Please wait while we load your content...
Please wait while we load your content...