Development and validation of an HPLC-FLD technique for colistin quantification and its plasma monitoring in hospitalized patients
Abstract
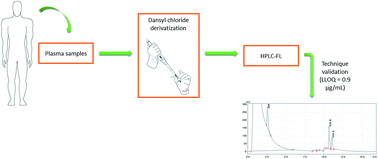
The increment of infections with multi-drug resistant gram-negative bacteria together with the high attrition rate of new antibacterial development programs has led to the renaissance of colistin as a new hope. However, the current administration of colistin to humans requires pharmacokinetic drug monitoring to individualize its posology, avoiding the development of resistant bacteria and the attainment of toxic concentrations. In this context, accurate, precise and selective methodologies are required to determine colistin plasma concentration. The present work is aimed at developing and fully validating a new high-performance liquid chromatography with fluorescence detection assay for the quantification of colistin in plasma samples of hospitalized patients. The chromatographic separation of colistin and an internal standard was achieved using a C18 column with a mobile phase comprised of acetonitrile and water. The detector was set at excitation/emission wavelengths of 343/500 nm and the retention time of the drug was shorter than those reported using other analytical techniques. The method was revealed to be linear in the concentration range of 0.09–9.00 μg mL−1 (which includes the therapeutic range of colistin), precise (coefficient of variance ≤ 6.4%), accurate (bias ≤ 14%) and selective. After full validation, the method successfully quantified the total colistin in plasma from patients treated with colistimethate sodium.


 Please wait while we load your content...
Please wait while we load your content...