Colorimetric detection of hydrogen peroxide and glucose using brominated graphene†
Abstract
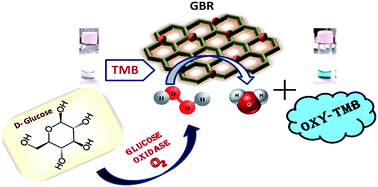
Very recently, we have reported a novel peroxidase mimetic material, brominated graphene (GBR) having ∼3% bromine content, which, in combination with H2O2 and 3,3′,5,5′-tetramethylbenzidine (TMB), has shown the property of S2− ion recognition (Anal. Chem., 2017, 89, 783–791). In the present work, we further have investigated the kinetic assay and colorimetric sensing ability of GBR towards hydrogen peroxide (H2O2) and glucose. The Michaelis–Menten constants (Km) and maximum initial velocities (Vmax) of GBR have been found to be 10.98 mM and 3.60 × 10−8 M s−1, respectively, for H2O2 and 0.83 mM and 0.68 × 10−8 M s−1, respectively, for TMB. A sensor combining TMB and GBR has been fabricated, which, upon addition to H2O2 or glucose with glucose oxidase solution at pH 4.48, showed colorimetrically a significant increase in the oxidation of TMB. The fabricated sensor system has displayed linearity for H2O2 and glucose estimation in the range 0.50–5.00 mM and 40–100 mM, respectively, and the corresponding limits of detection are found to be 0.417 and 28.41 mM, respectively. The present sensor system is also highly reproducible and selective. The results of real samples using this colorimetric method have been found to be comparable with the conventional auto-analyser method.


 Please wait while we load your content...
Please wait while we load your content...