Spectroscopy study of the interaction between endocrine disruptor 4-OH-2,2′,3,4′-BDE and human serum albumin
Abstract
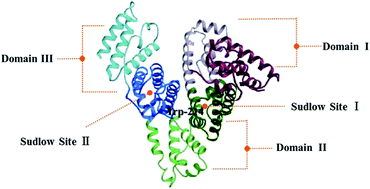
Hydroxylated polybrominated diphenyl ethers (OH-PBDEs), as one of the derivatives of polybrominated diphenyl ethers (PBDEs), have received extensive concern recently. In this paper, the interaction of OH-PBDEs and human serum albumin (HSA) was investigated using 4-OH-2,2′,3,4′-tetrabromodiphenyl ether (4-OH-BDE-42) as a representative. The binding constant, the number of binding sites and the binding site were investigated using fluorescence spectroscopy. The predominant forces of the interaction were van der Waals and hydrogen bonds, which was made clear by the calculation of thermodynamic parameters. The alterations of HSA protein secondary structure in the presence of 4-OH-BDE-42 were confirmed by UV-Vis and circular dichroism (CD) spectroscopy. The distance between HSA and 4-OH-BDE-42 was studied by Förster's non-radioactive energy transfer theory. An equilibrium dialysis experiment was performed, which indicated that 4-OH-BDE-42 severely affects the physiological function of HSA in transportation of 3,3′,5-triiodothyronine (T3), Trp and VB2. This paper has made deep investigation and detailed interpretation of the interaction of 4-OH-BDE-42 and HSA.


 Please wait while we load your content...
Please wait while we load your content...