Chiral determination and assay of optical isomers in clandestine drug laboratory samples using LC-MSMS
Abstract
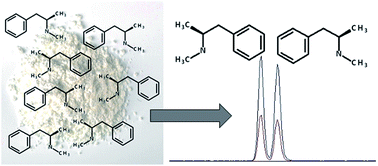
Many illicit drugs produced in clandestine laboratories exist as optical isomers, and due to their differing pharmacological properties, it is necessary to determine their respective amounts in a sample. Furthermore, the chemical signature of a clandestine laboratory sample could yield information about possible synthetic routes and product origin. The aim of this study was to optimise a method for the chiral resolution of amphetamine-type stimulants (ATS) and subsequently validate a liquid chromatography-tandem mass spectrometry (LC-MSMS) method for their simultaneous identification and quantification. This study used a CHIROBIOTIC V2 column, which contains interactive chiral additives, to successfully resolve the ATS enantiomers. The correlation coefficients (r2) acquired through the optimised method were determined to range from 0.9956 to 0.9989. Recovery was evaluated at two different concentrations and found to be between 78% and 102%. The method was proved to be fit for purpose as the repeatability and intermediate precision values were found to be below 2.40% and 5.18%, respectively. Five clandestine laboratory samples were analysed using the validated method and found to predominantly contain dextromethylamphetamine in concentrations ranging from 39.7 ppm to 5880 ppm. Dextroamphetamine was also identified in much smaller concentrations.


 Please wait while we load your content...
Please wait while we load your content...