A very fast 3-hydroxy-coumarin-based fluorescent probe for highly selective and sensitive detection of thiophenols and its application in water samples†
Abstract
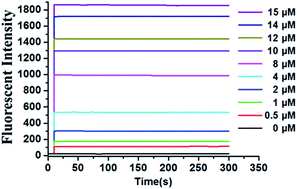
In this work, we have developed a novel off–on fluorescent probe by combining 7-diethylamino-3-hydroxy-coumarin as the fluorophore and 2,4-dinitrobenzenesulfonate as the recognition unit. This probe features a remarkable large Stokes shift (113 nm) and shows a highly selective and sensitive detection process for thiophenols with significant fluorescence turn-on responses. Biothiols, aliphatic thiols and reducing anions do not interfere with the sensing of thiophenols. Notably, it rapidly responds to various concentrations of PhSH and the fluorescence intensity reaches a plateau instantly. The limit of detection of the probe was found to be 7.3 nM in HEPES buffer (10 mM, pH 7.4) containing 30% THF as a co-solvent. The potential application of the probe was demonstrated by the quantitative detection of thiophenols in real water samples.

 Please wait while we load your content...
Please wait while we load your content...