Simultaneous determination of ginkgolides A, B and K in human plasma by UPLC-MS/MS and its application to the pharmacokinetic study of Ginkgo Diterpene Lactone Meglumine Injection in humans†
Abstract
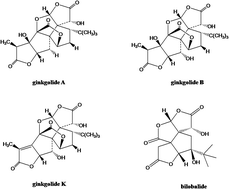
We developed and validated a rapid, selective and sensitive ultra-performance liquid-chromatography mass-spectrometry (UPLC-MS/MS) method for quantifying ginkgolide A, ginkgolide B and ginkgolide K as hydrolyzed forms in Ginkgo Diterpene Lactone Meglumine Injection (manufactured by Jiangsu Kanion Pharmaceutical Co., Ltd., Lianyungang, Jiangsu, China) for pharmacokinetic studies. Three ginkgolide compounds and internal-standard (IS), bilobalide, were extracted from human plasma by liquid–liquid extraction using ethyl acetate after acidification, and were separated on an Ace C18-AR column (2.1 mm × 50 mm, 3 μm) using a mobile phase of water containing 20 mM ammonium formate (adjusting pH to 5.4 using formic acid) and acetonitrile by gradient elution. Quantification was performed using negative-ion electrospray-ionization (ESI), and the content of ginkgolides was determined using multiple reaction monitoring (MRM) modes. Pharmacokinetics was assessed after intravenous infusion administration of Ginkgo Diterpene Lactone Meglumine Injection (containing 25 mg ginkgolides) to 3 healthy male Chinese volunteers. Analysis of the standard curves by linear regression revealed a high linearity over a 100-fold dynamic range for the three ginkgolides (the lower limit of quantitation values were 0.189–0.200 ng mL−1). The relative standard deviations of intra- and inter-day measurements were less than 10.7%, and accuracies were between −4.6% and 12.3% in terms of relative error; the extraction recoveries from human plasma were 89.4–91.9%, 81.0–85.0% and 78.5–82.5% for ginkgolide A, ginkgolide B and ginkgolide K, respectively. In conclusion, UPLC-MS/MS is rapid and sensitive for simultaneous quantification of multiple ginkgolides from Ginkgo Diterpene Lactone Meglumine Injection in human plasma and is suitable for pharmacokinetic studies.

 Please wait while we load your content...
Please wait while we load your content...