Chiral resolution with frozen aqueous amino acids†
Abstract
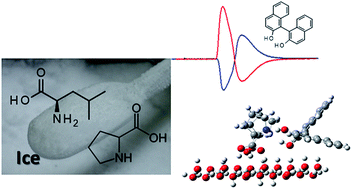
Frozen aqueous amino acids are screened to determine their chiral resolution using ice chromatography. Freezing allows convenient preparation of functional solid materials without organic syntheses. Frozen proline and leucine resolve the enantiomers of 1,1′-bi-2-naphthol (BINOL), while other amino acids tested do not show chiral selectivity. The effects seen when changing the pH of an amino acid solution before freezing suggest that the amino group of the amino acid plays an important role as a hydrogen bond acceptor. The molecular mechanism of chiral resolution is examined using Gaussian 9.0. A single proline molecule does not show chiral resolution capability. In contrast, two proline molecules fixed on the ice surface through the carboxyl groups show preferable interaction with the R enantiomer of BINOL. In the optimized structure of the Pro molecules on the ice surface, the distance between carboxyl oxygen and water oxygen on the ice surface ranges from 0.2533 to 0.2594 nm. The amino nitrogen atoms interact with the OH groups in BINOL with the distances of 0.2668 and 2.770 nm for the R-enantiomer and 0.2711 and 0.2717 nm for S-enantiomer. The preferable interaction with the R-enantiomer of BINOL is in accordance with the ice chromatography results, which reveal a stronger retentivity for the R enantiomer. These results strongly suggest that amino acids are expelled from the ice phase upon freezing, and form aggregates that provide multiple hydrogen bonding sites to enhance chiral resolution.

 Please wait while we load your content...
Please wait while we load your content...