Enhancing phosphate removal from water by using ordered mesoporous silica loaded with samarium oxide
Abstract
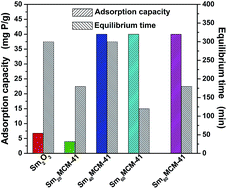
A series of ordered mesoporous silica loaded with samarium oxide (Sm-MCM-41) were synthesized by a facile one-step sol–gel route using hexadecyltrimethylammonium bromide (CTAB) as the template, tetraethylorthosilicate (TEOS) as the silica source, and hexahydrated samarium chloride as the precursor. The as-synthesized materials with the Sm/Si molar ratio ranging from 0.2 to 0.8 were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM), and N2 adsorption–desorption measurements. All obtained compounds possess an ordered hexagonal mesoporous structure with a high surface area, a large pore volume, and uniform pore size. The mesoporous composites were used as the novel adsorbents for phosphate ion (H2PO4−) removal from synthetic aqueous solutions. The phosphate removal capacity of Sm-MCM-41 with a Sm/Si molar ratio of 0.6 was up to 20 mg P/g. The Sm functionalized mesoporous silica materials show a higher phosphate removal capacity compared to MCM-41 and Sm2O3 particles, making them promising candidates for water quality control and protection.

 Please wait while we load your content...
Please wait while we load your content...