Validated LC-MS/MS method for simultaneous determination of Dasatinib and Sitagliptin in rat plasma and its application to pharmacokinetic study
Abstract
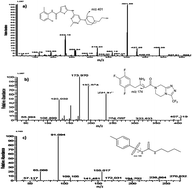
Dasatinib (DST), a tyrosine kinase inhibitor, is a novel anticancer agent and Sitagliptin (STG) is an antidiabetic agent of dipeptidyl peptidase-IV inhibitor class. A novel, sensitive and specific liquid chromatography tandem mass spectrometry (LC-MS/MS) based method has been developed for simultaneous monitoring of plasma levels of STG and DST in rat plasma. Both analytes and an internal standard (tolbutamide) were chromatographed on YMC-Pack ODS-AM (50 mm × 4.6 mm i.d., 3 μm) using a methanol : 2 mM ammonium acetate binary gradient mobile phase at a flow rate of 1 ml min−1 with a splitter (1 : 1) over a 5 min run time. Detection of analytes was performed on a LC-MS/MS system in multiple reaction monitoring (MRM) mode. The transitions monitored were 488.1 → 401.0, 408.1 → 174.0 and 271.1 → 155.0 for DST, STG and IS, respectively. The method was validated over a concentration range of 5.41–1029.60 ng ml−1 for DST and 5.64–1073.56 ng ml−1 for STG. The lower limit of quantification was 5.41 ng ml−1 and 5.64 ng ml−1 for DST and STG, respectively. Recoveries from spiked controls were >82% for the analytes and the internal standard at all QC levels. The intra- and inter-batch precision and accuracy across four quality control levels met established criteria of US Food and Drug Administration guidelines. This method was successfully applied to monitor the pharmacokinetic profile of both STG and DST in Wistar rats. This method can be applicable for pharmacokinetic drug–drug interaction studies.

 Please wait while we load your content...
Please wait while we load your content...