Enhanced spectrophotometric methods for trace metal determination in waters: zinc as an example
Abstract
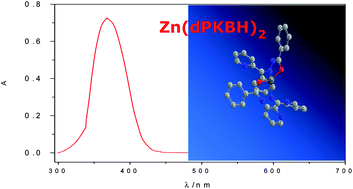
Although spectrophotometric methods seem to be outdated as analytical tools for the analysis of trace metals in waters, there is no doubt that they still present a series of advantages over other advanced techniques (simplicity, speed, low cost and maintenance, portable instrumentation, etc.). Even more, if experimental conditions are strictly controlled, it is possible to develop highly competitive methods. In this sense, method validation turns into a key feature to improve and demonstrate its applicability. Bearing this in mind, a simple and very sensitive spectrophotometric method for the direct determination of μg L−1 levels of zinc in natural waters has been developed and in-house validated. It is based on the reaction of Zn(II) with

 Please wait while we load your content...
Please wait while we load your content...